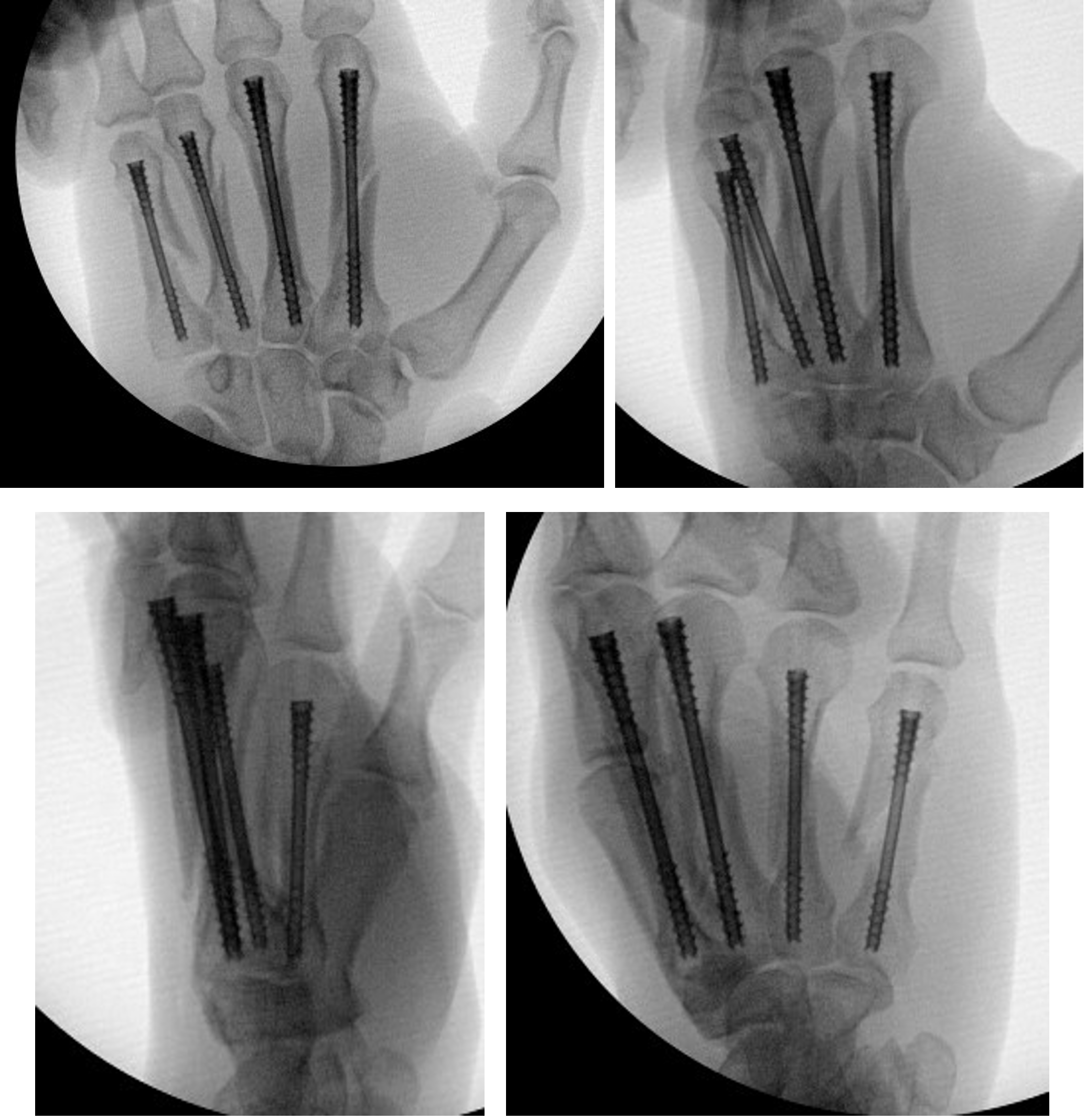
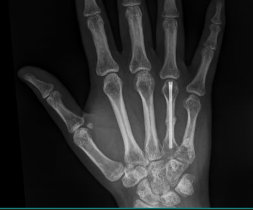
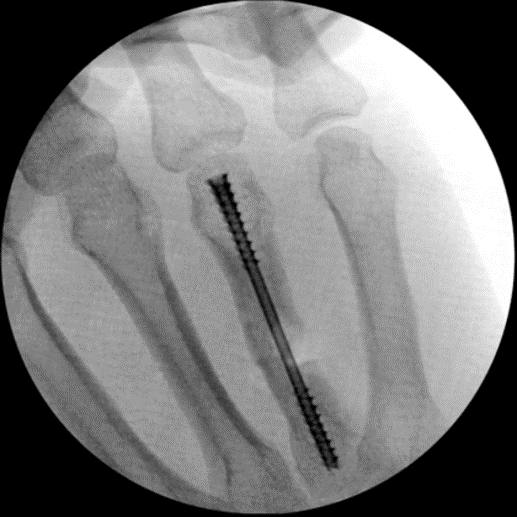
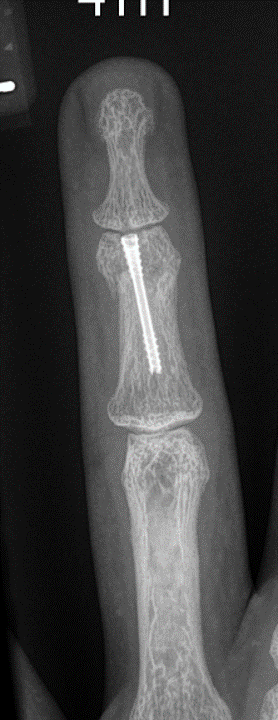
Fracture Management
Field Orthopaedics is committed to supporting the continued improvement of clinical practice. We appreciate that one of the best ways to do this is through the sharing of case reports. Our case report program provides a collection of user experiences with the Micro Screw and NX Nail across a variety of indications.
If you are interested in contributing to the program, please click on the button below to view the process and get in touch either directly via marketing@fieldorthopaedics.com or your local representative.
FOR HEALTHCARE PROFESSIONALS ONLY.
It’s important to note that, like any medical device, the NX Nail and Micro Screw may not be suitable for all patients and there are potential risks associated with the procedure. We encourage healthcare professionals to carefully consider individual circumstances and consult with medical experts to determine the best treatment options. Transparency and patient safety are our top priorities, and we’re committed to providing accurate information and supporting our customers every step of the way.
For further information on limitations and potential risks please access product specific IFU’s.
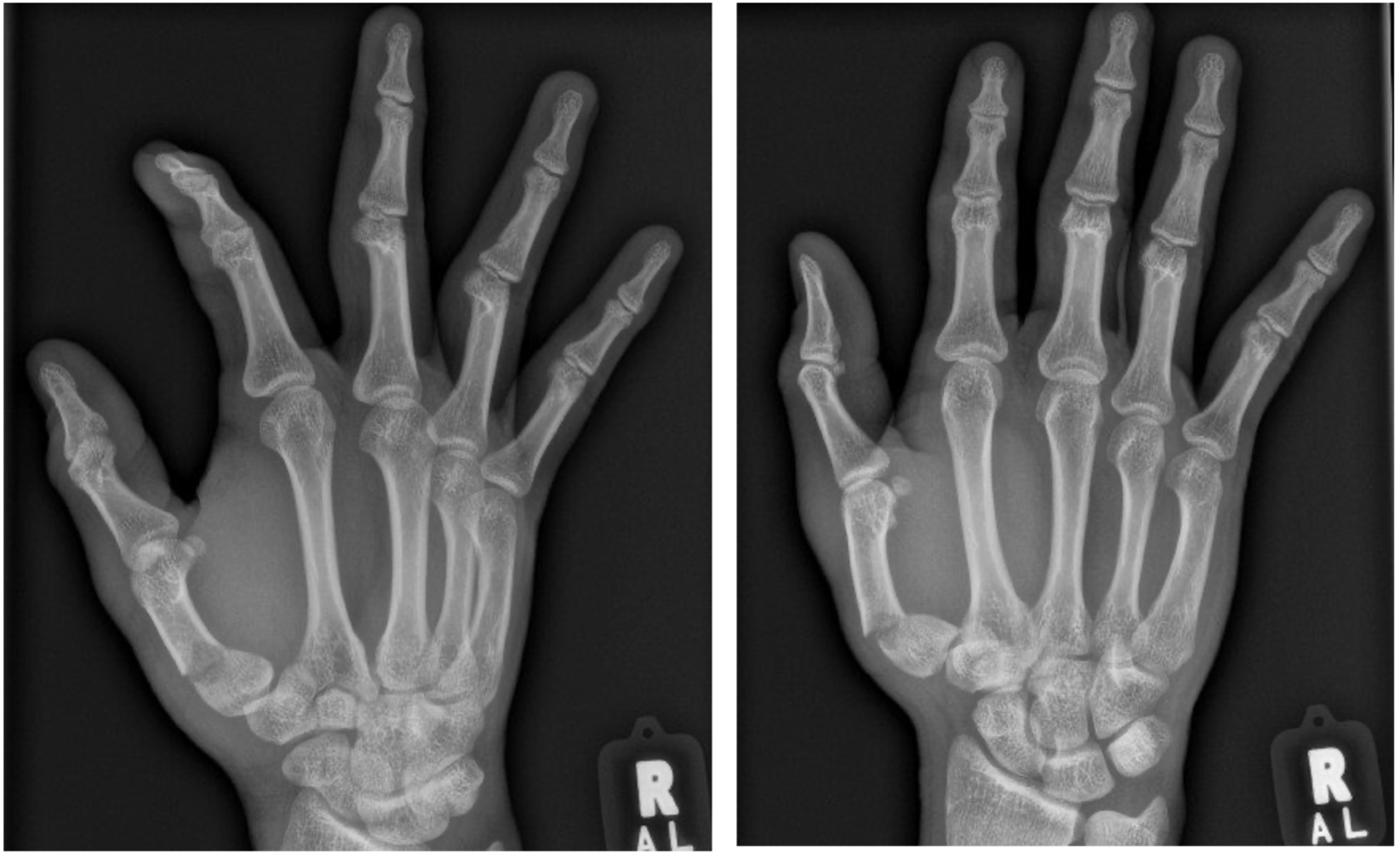
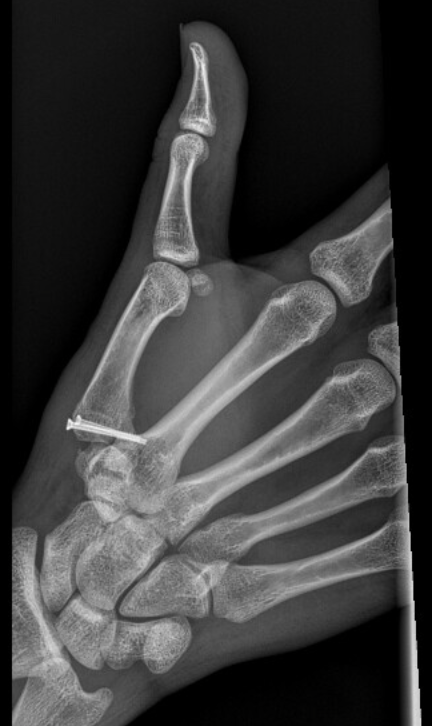
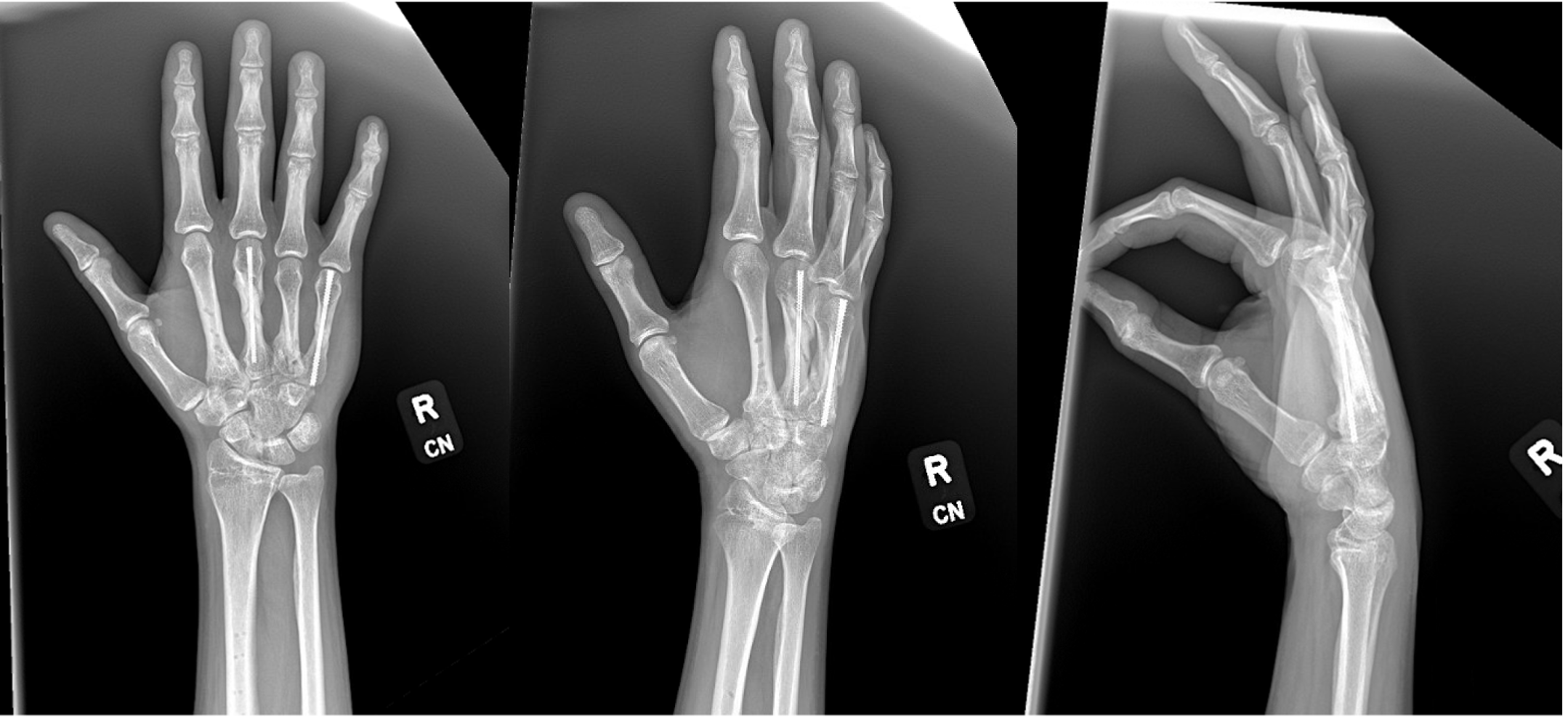
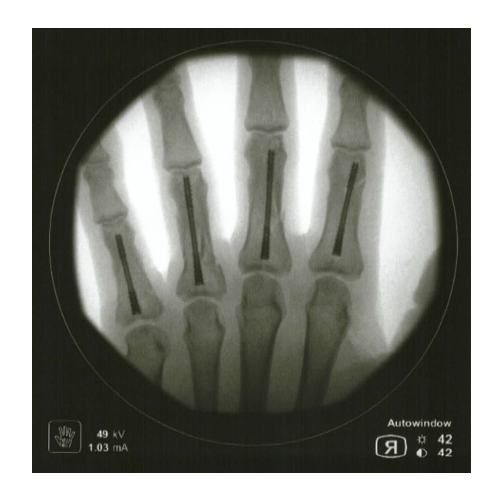
Case Reports - Field Orthopaedics
What are the risks associated with intramedullary nailing?
Contraindications, potential complications, warnings and precautions:
In any surgical procedure, the potential for complications and adverse reactions exists. Contraindications include cases of inflammation, cases of active or suspected sepsis / infection and osteomyelitis, patients with certain metabolic diseases and applications that are not defined by the indications.
The risks and complications with these implants can include loosening, deformation or fracture of the implant, acute post-operative wound infections and late infections with possible sepsis, thrombosis and embolism, wound hematoma and delayed wound healing, temporary and protracted functional neurological perturbation, tissue reactions resulting from allergy or foreign body reaction to dislodged particles and corrosion with localised tissue reaction and pain. All complications listed here are not typical of the Field Orthopaedics (FO) Bony Trauma Extremity System (BTES) but can be in principle observed with any implant.
Warnings and precautions related to the use of the FO BTES include; re-operation to remove or replace implants may be required at any time due to medical reasons or device failure, if corrective action is not taken complications may occur; use of an undersized screw/nail in areas of high functional stresses may lead to implant fracture and failure; plates and screws, wires, or other appliances of dissimilar metals should not be used together in or near the implant site; the FO Screws, NX Nails, Plates, Pins and K-Wires, and FO drill bits are intended for single use only, re-use may cause product failure and could lead to disease transmission; instruments, guide wires and screws/nails are to be treated as sharps; Field Orthopaedics branded instrumentation is recommended for use in conjunction with FO BTES Implants.
These devices have not been evaluated for safety and compatibility in the MR environment. For further details, please consult the instructions for use.