Griplasty System Base of Thumb with Needles
Next Generation CMC Suspensionplasty
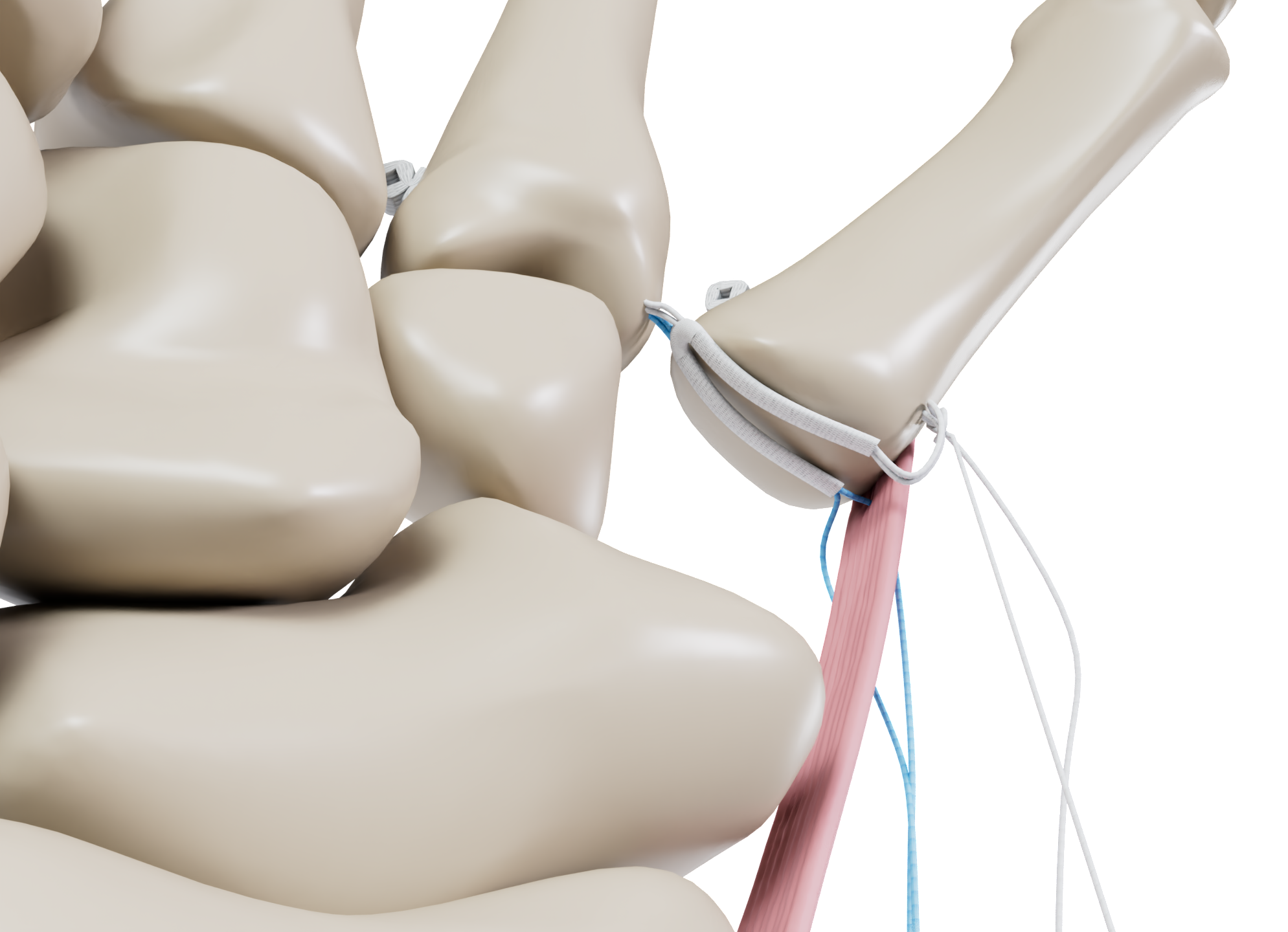
The GriplastyTM System Base of Thumb (BoT) with Needles is designed to provide a solution for patients with advanced-stage first carpometacarpal (CMC) joint osteoarthritis that relieves pain and restores thumb stability and strength.1,2
The GriplastyTM BoT procedure requires only a single incision allowing for either a volar or dorsal approach. The unique V-Sling of the GriplastyTM System Base of Thumb is designed to mitigate concerns of proximal migration of the first metacarpal by providing a broader surface area of support compared to traditional systems.2
The innovative deployment guidance* of the GriplastyTM System is designed to enable a precise, accurate and reproducible technique.2 The anchors are deployed on the far cortex, above the area of degenerative bone, which is designed to increase the pull-out strength of the construct.2,3,4
*Patent pending. Product may not be available in all markets. Please contact Field Orthopaedics if you have any questions about product availability in your area.

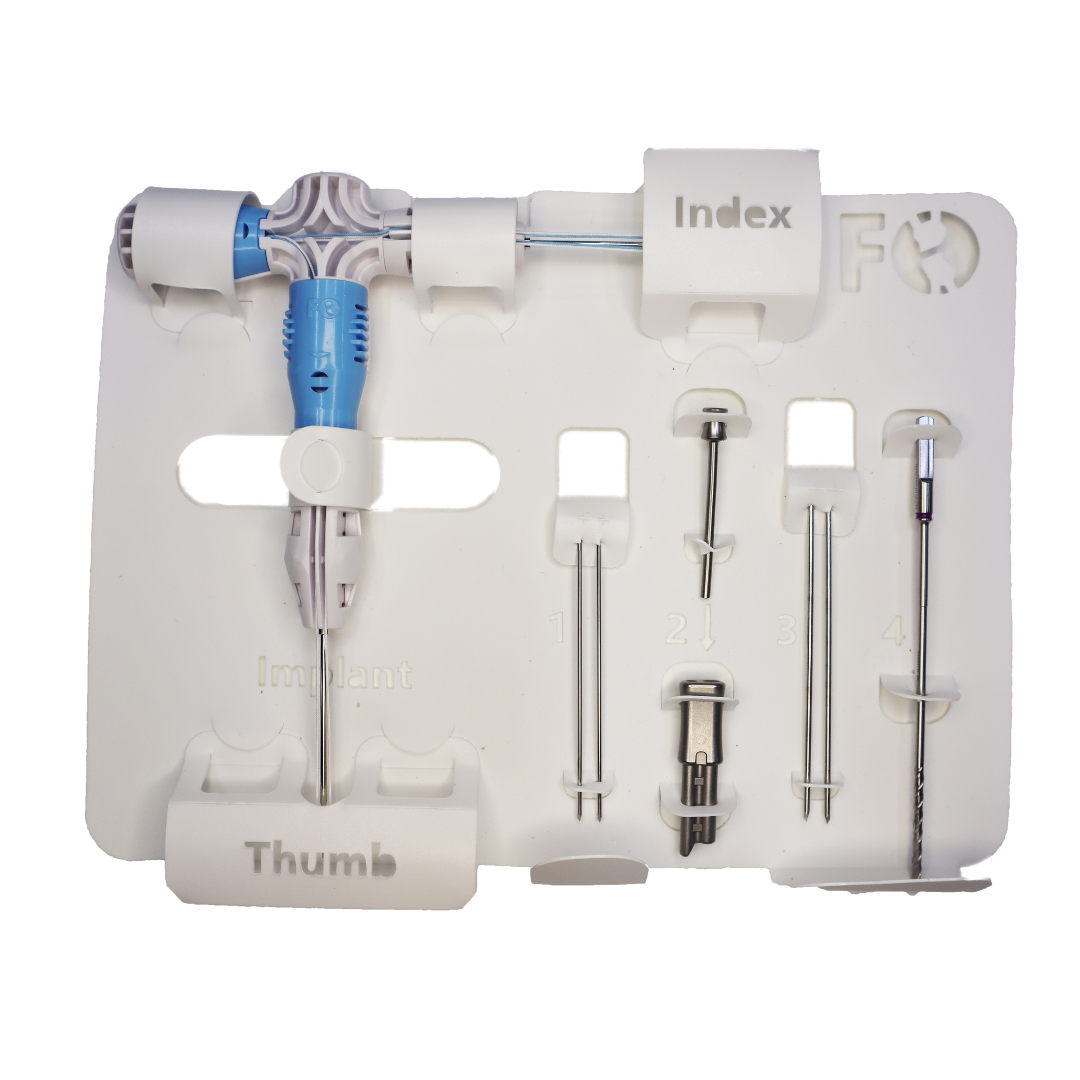
Benefits of sterile single-use instruments.
Strong Low Profile Suture Anchors
- Minimizing soft tissue irritation.6
- Providing reliable fixation.
- High pullout resistance with far cortex engagement.3
- Pre-loaded for quick insertion.
Streamlined Delivery
- Precise and pre-determined drill path.2
- Guided and accurate anchor placement.2,5
- Reproducible and consistent anchor deployment.2
- Anatomically contoured leading tip designed to improve intra operative positioning.2
Sophisticated All-in-One System
- Pre-loaded and always one step ahead.
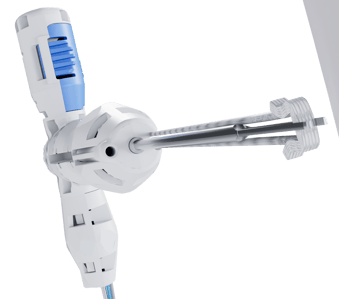
- Ergonomically designed T-Handle specifically tailored for the upper limb surgeon.2
- Streamlined and efficient workflow.7
- Clearly labeled for intuitive use.
- Reliable product performance with new instruments for every case.8
- Environmentally conscious and cost efficient single use packaging to optimize the perioperative process.9,10,11,12
Why choose the GriplastyTM System?
The introduction of terminally sterilized GriplastyTM System Base of Thumb with Needles allows for clinical variations in the management of CMC joint arthritis. From being a seamless addition to your trapeziectomy procedures, to facilitating optional tendon transfers, the GriplastyTM System Base of Thumb with Needles provides a solution for you and your patients.2,5
Versatile and Innovative Solution
- Single incision site supporting a volar or dorsal approach.5
- Reproducible technique with deployment guidance instrumentation.2
- Implantation above the area of degenerative bone reduces risk of failure.4
Precise and Definitive Construct
- Intuitive, easy to use, tensioning construct.2
- Unique V-sling with increased surface area of support designed to create a stronger construct.2
- Facilitates tendon transposition if clinically required.5
.

What are the risks associated with a GriplastyTM CMC Suspensionplasty?
Contraindications, potential complications, warnings and precautions:
In any surgical procedure, the potential for complications and adverse reactions exists. Contraindications include cases of inflammation, cases of active or suspected sepsis / infection and osteomyelitis, patients with certain metabolic diseases and applications that are not defined by the indications.
The risks and complications with these implants can include loosening, deformation or failure of the implant, acute post-operative wound infections and late infections with possible sepsis, thrombosis and embolism, wound hematoma and delayed wound healing, temporary and protracted functional neurological perturbation and tissue reactions as the result of allergy or foreign body reaction to dislodged particles.
All complications listed here are not typical of the Field Orthopaedics (FO) Extremity All Suture System (EASS) but are in principle observed with any implant.
Warnings and precautions related to the use of the EASS include; Re-operation to remove or replace implants may be required at any time due to medical reasons or device failure. If appropriate action is not taken, complications may occur; Use of an undersized anchor in areas of high functional stresses may lead to implant fracture and failure; All implants and instrumentation in the EASS are intended for single use only; re-use may cause product failure and could lead to disease transmission; Use of undersized/oversized drill/K-wire to generate anchor pilot hole may lead to implant failure fixation. Use drill and K-wire supplied with the implant; FO branded instrumentation is recommended for use in conjunction with EASS implants; Postoperatively, until healing is complete, the fixation provided by this device should be protected. The postoperative regimen prescribed by the surgeon should be strictly followed to avoid adverse stresses being applied to the implant; Detailed instructions on the use and limitations of the device should be given to the patient; Any decision to remove the device should take into consideration the potential risk to the patient of a second surgical procedure. Implant removal should be followed by adequate postoperative management; Preoperative and operating procedures, including knowledge of surgical techniques and proper selection and placement of the implant, are important considerations in the successful utilization of this device; Do not re-sterilize this device; Do not use beyond the expiration date listed on the label. The performance, safety, and/or sterility of the device cannot be assured beyond the expiration date; Remove items from sterile packages using aseptic techniques; Avoid excessive impaction during insertion as this may lead to inserter damage and/or breakage. If insertion resistance is encountered, do not impact harder. Replace the implant and repeat the drilling/insertion procedure; Visually inspect the inserter for potential bending, damage or breakage after each insertion; Instruments, and components such as K-wires, Drills, Anchor Holders and needles are to be treated as sharps; Instrument must be disposed of according to hospital policy and procedure.
These devices have not been evaluated for safety and compatibility in the MR environment. For further details, please consult the instructions for use.
References
- Larsen CG et al. Knotless suture anchor suspensionplasty for basal joint arthritis: a prospective case series with short-term outcomes. Bull Hosp Jt Dis.2022;80(2):129-3
- Data on file.
- Hozack et al. Optimal Position of the Bone Anchor for the Internal Brace Suspensionplasty Technique for Thumb Basal Joint Arthroplasty. J Hand Surg Am. 2024 Apr;49(4):380.e6.doi:10.1016/j.jhsa.2022.08.001. Epub 2022 Sep 10. PMID: 36100487.
- Oh J H et al. Pullout Strength of All-Suture Anchors: Effect of the Insertion and Traction Angle - A Biomechanical Study. Arthroscopy. 2018 Oct;34(10):2784-2795 doi:10.1016/j.arthro.2018.04.028. Epub 2018 Sep 1. PMID: 30181056.
- Field Orthopaedics. (2024). GriplastyTM Surgical Technique. Brisbane, Australia: Field Orthopaedics.
- Ergun S et al. The Clinical and Biomechanical Performance of All-Suture Anchors: A Systematic Review. Arthroscopic Sports Medicicine and Rehabilitation. 2020 May;28;2(3);e263-e275. doi: 10.1016/j.asmr.2020.02.007. PMID: 32548592; PMCID:PMC7283965.
- Wong J et al. Delays in the Operating Room: Signs of an Imperfect System. Canadian Journal of Surgery. 2010 Jun;53:189-194.
- Hale L. Developing Devices to Meet Today's Orthopaedic Trends - An Orthopaedic Innovators Q&A (ed.) Sean Fenske. Orthopaedic Design & Technology, Oct 28, 2022.
- Leiden A et al. Life Cycle Assessment of a Disposable and a Reusable Surgery Instrument Set for Spinal Fusion Surgeries. Resources, Conservation & Recycling. 2020;156
- Yasser, A. Value Based Healthcare: Maximizing Efficacy and Managing Risk with Spinal Implant Technology. Interdisciplinary Neurosurgery. 2020;22.
- Agarwal A et al. A Paradigm Shift Toward Terminally Sterilized Devices. Clinical Spine Surgery. 2018;31:308-311.
- Goldberg, TD et al. Logisitical and Economic Advantages of Sterile-Packed, Single-Use Instruments for Total Knee Arthroplasty. Journal of Arthroplasty. 2019;34:1876-1883

.gif)
