MEET THE SURGEON

Dr. Genghis Niver, MD
Orthopaedic SurgeonSummit Health
Florham Park, NJ
DISCLAIMER
The opinions expressed by Dr. Niver are those of Dr. Niver and not necessarily those of Field Orthopaedics. Individual experiences may vary.
The procedure described in the report may differ from the manufacturer’s surgical technique. Surgeons are advised to review the product specific surgical technique prior to performing any surgery.
A surgeon must always rely on their own professional clinical judgement when deciding whether to use a particular product when treating a particular patient. A surgeon must always refer to the instructions for use, product label and surgical technique before using any Field Orthopaedics product. Product may not be available in all markets. Please contact your Field Orthopaedics representative if you have any questions about availability of Field Orthopaedics products in your area.
OVERVIEW
Patient is a 66-year-old right hand dominant female who injured her right small finger after suffering a fall while walking her dog. Due to the angulation present, surgery was offered in the form of a percutaneous reduction and internal fixation with a Field Orthopaedics NX Nail.
CASE INTRODUCTION
The patient is a 66-year-old right hand dominant female who injured her right small finger after suffering a fall while walking her dog. Radiographs confirmed a non-displaced but angulated right small proximal phalanx base fracture.
CASE PRESENTATION
On examination, there was global swelling of the right hand with tenderness of the right small proximal phalanx base. She had no malrotation at resting posture and was unable to make a fist. She had no prior injury or pain in this finger.
Below: Pre-Operative Imaging

SURGICAL APPROACH
The patient underwent surgical treatment 3 days after the injury. The procedure took place under general anesthetic with the patient in supine position.
Reduction
Closed reduction was achieved with axial traction and volar translation of the distal portion of the proximal phalanx. The reduction was verified with mini c-arm imaging. To confirm the correct width of the implant, a template was placed to match the isthmic fit as closely as possible using mini c-arm imaging.
Below: Isthmic Sizing Imaging

Fixation
Afterwards, the metacarpophalangeal joint was flexed while maintaining axial traction of the middle phalanx. The K-wire was inserted from the ulnar portion of the proximal phalanx base across the fracture into the distal portion of the proximal phalanx in an anterograde fashion. A second K-wire was inserted from the radial portion of the proximal phalanx base into the distal portion in a non-crossing manner. These K-wires were placed into the distal portion of the proximal phalanx just short of the proximal interphalangeal (PIP) joint. No further malrotation or angulation was noted of the fracture.
A nick incision was made using an 11-blade scalpel around the K-wires and scissors were used to bluntly spread the tissue down to the proximal cortex. Using a depth gauge, both K-wire lengths were measured. The K-wires were then placed more distal into the PIP joint to prevent further pullout. 3-4 mm were subtracted from the measured length of the K-wire and the overdrill was placed for a Field Orthopaedics 2.0 mm NX Nail. The Extended Meta Drill was then placed into the proximal fragment. A 26 mm and 28 mm Nail was then placed over the K-wire and it was noted that the reduction was maintained with no further angulation or malrotation. The nails were advanced past the proximal cortex of the proximal phalanx. The K-wires were then removed. 4-0 Vicryl Rapide sutures were used close the nick incision with a simple interrupted suture. An adhesive glue was then applied, with gauze and compressive wrap applied.
Below: Intra-Operative Imaging

POSTOPERATIVE PROTOCOL
The patient remained in a soft dressing for two weeks and then referred to a hand therapist for full range-of-motion with a 10 lb weight restriction.
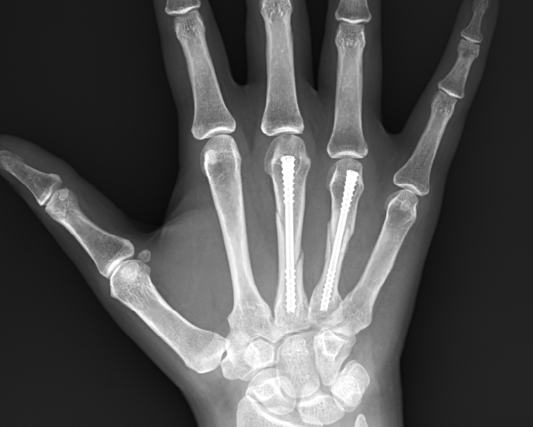
Below: Post-Operative Imaging

FOLLOW UP
At ten weeks post-operation, the patient demonstrated excellent clinical progress. She achieved full range of motion in her right small finger with no pain or functional limitation. Radiographs showed complete fracture healing with maintained alignment. The patient had returned to all normal activities, including dog walking, with no restrictions. No complications were observed during the healing process.
Below: Ten-week Images


CONCLUSION
This case demonstrates the successful application of the Field Orthopaedics NX Nail system in treating an angulated proximal phalanx base fracture. The minimally invasive technique, combined with stable fixation and early mobilization, resulted in excellent radiographic and functional outcomes at 10 weeks post-operation.
Product Resources
Field Orthopaedics. (2024). NX Nail Phalanx Long Surgical Technique. Brisbane, Australia: Field Orthopaedics.
FO-010398-MM Version 2 April 2025